Kossel-Lewis Approach to Chemical Bonding
Kossel-Lewis Approach to Chemical Bonding: Overview
This topic covers concepts, such as, Atom, Chemical Bonds, Lewis Structure for Simple Molecules & Electron Deficient Species etc.
Important Questions on Kossel-Lewis Approach to Chemical Bonding
What are the formal charges on and in the Lewis (electron dot) structure of the phosphate oxyanion represented in the figure?
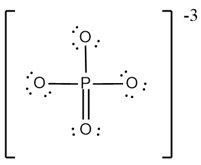
Electron deficient species among the following is:
Which of the following oxides is an odd-electron molecule.
Which of the following noble gas compound has hybridisation?
If an element is represented by its Lewis symbols as 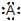 , then A may be
, then A may be
What will be the probable chemical formula if the valence shell of element contain three electrons, while the valence shell of element contain six electrons are combined?
In molecule, the number of bond pairs and lone pairs of electrons are .
In the Lewis structure, the formal charge on the central atom of is
Maximum number of lone pairs is present in Lewis dot structure of which compound?
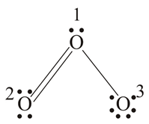
In ion formal charge on the oxygen atom of bond is
The formal charge on the -atoms in the ion is
In which of the following molecule central atom is having complete octet?
Which of the following is not an application of formal charge?
Lewis symbols are used to depict _____ in simple molecules.
The number of dots around the symbol represents the number of _____ electrons.
In molecule, the number of bond pairs and lone pairs of electrons are:
Which of the following is a Super octet molecule?
Metals lose electrons during ionization. This change is called
Assertion. is not a stable molecule.
Reason. A stable molecule must have 8 electrons around the central atom, i.e., octet rule should be satisfied.
